A Typical 3D Cell Culture Workflow
While specific protocols for 3D cell culture growth vary, certain universal aspects exist. These include:
Before Starting
Find out more about planning your experiment.
1. Sample Preparation and 3D Cell Culture
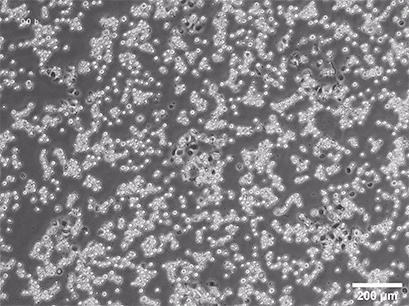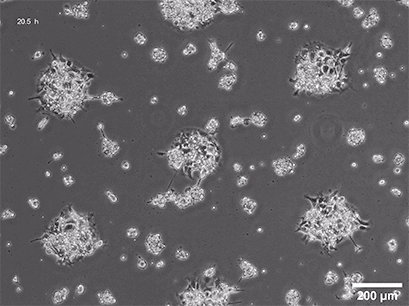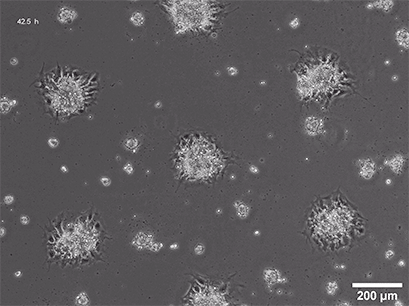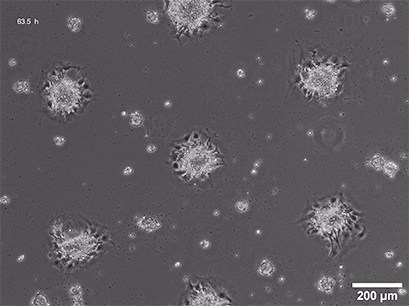
Initiating a 3D culture starts with isolating cells through mechanical and enzymatic digestion. Choosing appropriate enzymes and conditions is crucial for effective cell dissociation and viability, but factors such as processing time and ambient temperature are also critical. The process typically involves tissue separation with sterile scissors, enzymatic dissociation with collagenase, and homogenization. The aim is to achieve a uniform single cell suspension, often involving purification through Fluorescence-Activated Cell Sorting (FACS) or Magnetic-Activated Cell Sorting (MACS). Alternatively, cells for spheroid generation, like cancer cell lines, can be sourced from suppliers. It is usually best to cultivate 3D cells immediately, though protocols exist for freezing specific cell types.
Several methods for the aggregation and cultivation of 3D cells exist. While spheroid cultivation follows heavily standardized protocols, organoid cultivation often requires a more careful revision of available procedures. In addition to these scaffold-free approaches, scaffold-based 3D cell culture models are also widely used. These models employ biocompatible scaffolds that provide a supportive matrix where cells can attach, grow, and organize into three-dimensional structures.
Generation and Culture of 3D Cell Models
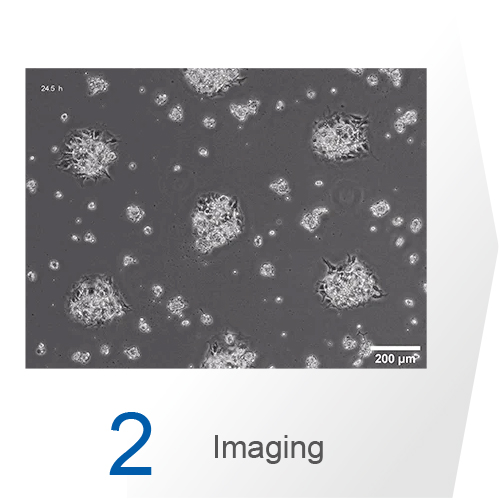
The choice of the most suitable imaging method often depends on the research question, available resources, and expertise. While phase contrast microscopy is sufficient for viability testing, complex antibody staining for fluorescence microscopy is often used to analyze the architecture and composition of cell aggregates. For getting optimal images, detailed planning and optimization of the fixation and staining protocol is crucial. Confocal microscopy provides high-resolution optical sectioning to elucidate internal structures of organoids and spheroids. Multiphoton microscopy offers deeper tissue penetration and reduced phototoxicity, making it ideal for imaging thicker specimens and long-term live cell imaging.
ibidi Solutions for 3D Cell Culture Assay Imaging
Typical readouts from 3D cell culture experiments include analyzing both secreted and intracellular biomolecules, but also assessing viability, growth, differentiation, and interactions with other cells, organisms, or compounds. Various quantitative downstream analyses include metabolic measurements, toxicology screens, transcriptomic profiling, and many more. Protocols exist for isolating biomolecules even from small cell quantities, while imaging readouts often require more meticulous preparation and specialized equipment.
Effective planning is essential for the success of 3D cell culture experiments. This process begins by setting clear research objectives and selecting the appropriate cell types. The choice of protocols for cell isolation and culture is critical, and these protocols should be continuously refined. Protocol adjustments include optimizing media composition, incubation temperature, and CO2/O2 levels, along with selecting the most suitable matrix for 3D growth. Moreover, integrating advanced cultivation methods such as dynamic flow conditions and exploring multi-organ-crosstalk using organ-on-a-chip systems or complex bioprinting approaches can further enhance experimental design.
The choice between organoids and spheroids should align with the research question, available resources, and established protocols. Organoids provide complex, organ-like structures suitable for comprehensive tissue studies but demand more specialized expertise and resources. Spheroids, being simpler, are essential in tumor research and high-throughput drug screening, and often allow more cost-effective approaches.

Spheroid of human intestinal fibroblasts and endothelial cells sprouting in a fibrin hydrogel cultured in a µ-Slide 4 Well. Endothelial cells were stained with anti-CD31 (yellow), fibroblasts with anti-vimentin (red), and nuclei with DAPI (cyan). Confocal image by H. Nogueira Pinto, Bioengineered 3D Microenvironments Group, University of Porto, Portugal.
Ultimately, it is also important to plan for practical readouts, including data collection and analysis, as these can influence culture methods and conditions. A key factor in obtaining high-quality readouts is the compatibility of the cell culture surface with imaging techniques. Traditional surfaces often either pose challenges in cell adhesion or optical clarity, affecting the efficacy of advanced imaging methods. Complementing this, the ibidi µ-Slides, µ-Dishes, and µ-Plates feature tissue culture-treated (ibiTreat) coverslip bottoms with excellent optical quality. These products are optionally available with various surfaces, and can be coated in a similar process to standard plastic labware while fully retaining image quality.