Advanced Cultivation of 3D Cell Models
Advanced cultivation techniques, such as dynamic flow culture of 3D cells, co-cultures and bioprinting, offer enhanced environmental control and complexity for more in vivo-like approaches. These advancements mark a substantial leap from traditional cultivation methods, offering enhanced realism and potential in 3D cellular studies.
- Dynamic Flow Culture of 3D Cells
- Spheroid Aggregation on a µ-Pattern Under Flow
- Co-Cultivation of Multiple 3D Cell Types
- Bioprinting of Complex Tissue-Like Structures
Dynamic Flow Culture of 3D Cells
Dynamic flow culture represents an important advancement in 3D cell culture technology. This method involves the continuous flow of media over cell aggregates, closely mimicking the dynamic fluid movement found in living organisms. This constant flow ensures an efficient supply of nutrients and oxygen while facilitating the removal of waste products, crucial for the long-term viability and functionality of 3D cell clusters. This technique is especially valuable in drug testing, disease modeling, and tissue engineering, where accurate simulation of the in vivo environment is essential. On the downside, establishing a dynamic flow culture requires sufficient financial resources and expertise in handling.
To establish a dynamic flow culture system, generated 3D structures are first seeded in a suitable microfluidic chip or culture chamber. This setup is then connected to a pump system, such as the ibidi Pump System, enabling controlled media flow. This configuration allows for the precise manipulation of flow characteristics, such as speed and pattern.
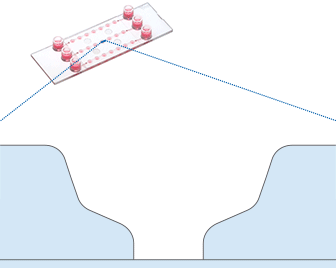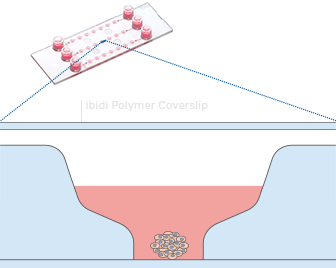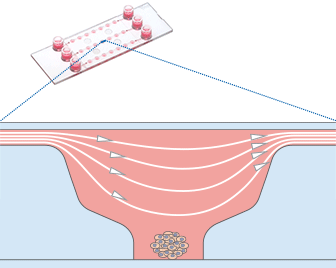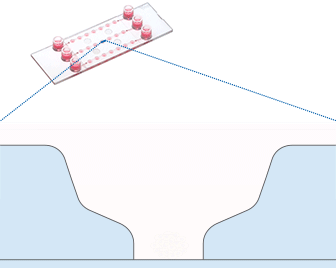
Working principle of the ibidi µ-Slide Spheroid Perfusion, which can be connected to a pump system (e.g., the ibidi Pump System) for long-term cultivation of spheroids.


L929 fibroblasts show spheroid formation in the µ-Slide Spheroid Perfusion, Bioinert, days 1–14, seeding concentration 5 x 105 single cells/ml. Left: no perfusion, medium exchange every second day. Right: perfusion with the ibidi Pump System, 0.75 ml/min. Phase contrast microscopy, 10x objective lens, well diameter 800 µm.
ibidi Solutions for the Dynamic Flow Culture of 3D Cells
Selected Publications for Dynamic Flow Culture of 3D Cells
Human tenon’s capsule fibroblasts (HTCF) were cultivated in 3D in a hydrogel under flow using the µ-Slide I Luer.
Fung M, Armstrong JJ, Zhang R, Vinokurtseva A, Liu H, Hutnik C. Development and Verification of a Novel Three-Dimensional Aqueous Outflow Model for High-Throughput Drug Screening. Bioengineering (Basel). 2024;11(2):142. doi:10.3390/bioengineering11020142.
Read article
Mesenchymal stromal cell (MSC) adhesion and transmigration studies were performed under shear stress conditions in the µ-Slide I Luer 3D.
Ye T, Liu X, Zhong X, Yan R, Shi P. Nongenetic surface engineering of mesenchymal stromal cells with polyvalent antibodies to enhance targeting efficiency. Nat Commun. 2023;14(1):5806. doi:10.1038/s41467-023-41609-8.
Read article
Dynamic cell culture of heart and kidney organoids was performed using the ibidi Pump System and the µ-Slide III 3D Perfusion.
Gabbin B, Meraviglia V, Angenent ML, et al. Heart and kidney organoids maintain organ-specific function in a microfluidic system. Mater Today Bio. 2023;23:100818. doi:10.1016/j.mtbio.2023.100818.
Read article
Dynamic cell culture of human ovarian cortical tissue was performed using the using the ibidi Pump System and the µ-Slide III 3D Perfusion.
Del Valle JS, Mancini V, Laverde Garay M, et al. Dynamic in vitro culture of cryopreserved-thawed human ovarian cortical tissue using a microfluidics platform does not improve early folliculogenesis. Front Endocrinol (Lausanne). 2022;13:936765. doi:10.3389/fendo.2022.936765.
Read article
Co-Cultivation of tumor spheroids was done in the µ-Slide III 3D Perfusion in serial connection with HUVEC cells in the µ-Slide I 0.8 Luer.
Algarni A, Greenman J, Madden LA. Doxorubicin Enhances Procoagulant Activity of Endothelial Cells after Exposure to Tumour Microparticles on Microfluidic Devices. Hemato. 2020; 1(1):23-34. doi:10.3390/bloods1010006.
Read article
Spheroid Aggregation on a µ-Pattern Under Flow
The ibidi µ-Patterning technology merges adherent spots with a Bioinert surface and thus enables precise control over the spatial and dimensional properties of the aggregate growth. This structure promotes important cell-cell interactions for the formation of 3D cell aggregates. The technology's unique capability to manipulate pattern dimensions and geometries allows for the creation of spatially defined structures. Consequently, researchers can produce spheroids with consistent size, shape, and cell distribution, enhancing uniformity and experimental reproducibility.
The µ-Slide VI 0.4 With Multi-Cell µ-Pattern allows for the simultaneous generation of multiple spheroids, with a capacity of up to 180 spheroids in one channel. This translates to the potential of producing approximately 1080 spheroids per µ-Slide. This feature not only increases throughput but also ensures consistency and precision in spheroid formation, crucial for advanced cell culture experiments.
Additionally, the channel design of the µ-Slide VI 0.4 is specifically engineered to accommodate integration into perfusion systems. This feature facilitates continuous media exchange and long-term cultivation, essential for creating physiological conditions that closely mimic the in vivo environment. These channels are capable of generating a defined shear stress, further improving cultivation conditions and reflecting more realistic physiological scenarios. This is central to advanced cellular studies, where maintaining and studying cells under dynamic, lifelike conditions is critical for accurate and relevant results.

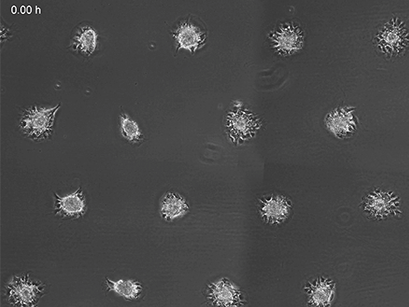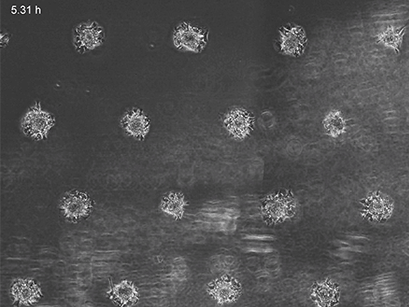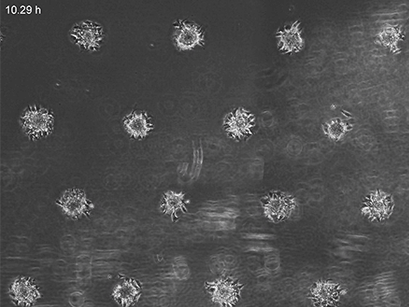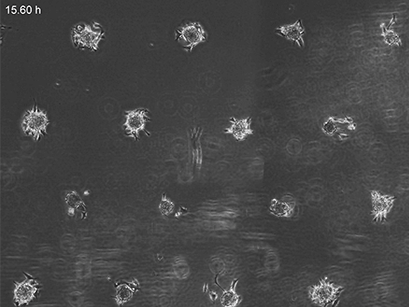
3T3 fibroblasts were seeded on a patterned µ-Slide VI 0.4. A shear stress of 3 dyn/cm2 was applied 7 days after cell seeding. 15 h time lapse microscopy, 4x objective.
ibidi Solutions for Spheroid Aggregation on a µ-Pattern Under Flow
Co-Cultivation of Multiple 3D Cell Types
By combining different cell types in 3D-structures, co-culture systems offer a more accurate representation of the in vivo cellular environment. These systems enable cell-cell and cell-matrix interactions, as well as chemotactic transmigration and penetration, closely mimicking the complexity of living tissues. This approach is particularly advantageous in studying tumor microenvironments, tissue regeneration, and disease modeling. It allows for the observation of how different cell types influence each other in a spatially relevant context. Additionally, 3D co-cultures provide a more dynamic and physiologically relevant model for drug screening and toxicity testing, leading to more reliable and translatable outcomes in biomedical research. However, 3D co-cultures often require even more complex protocol adjustments since optimal cultivation and experiment conditions must be established for several cell types simultaneously.
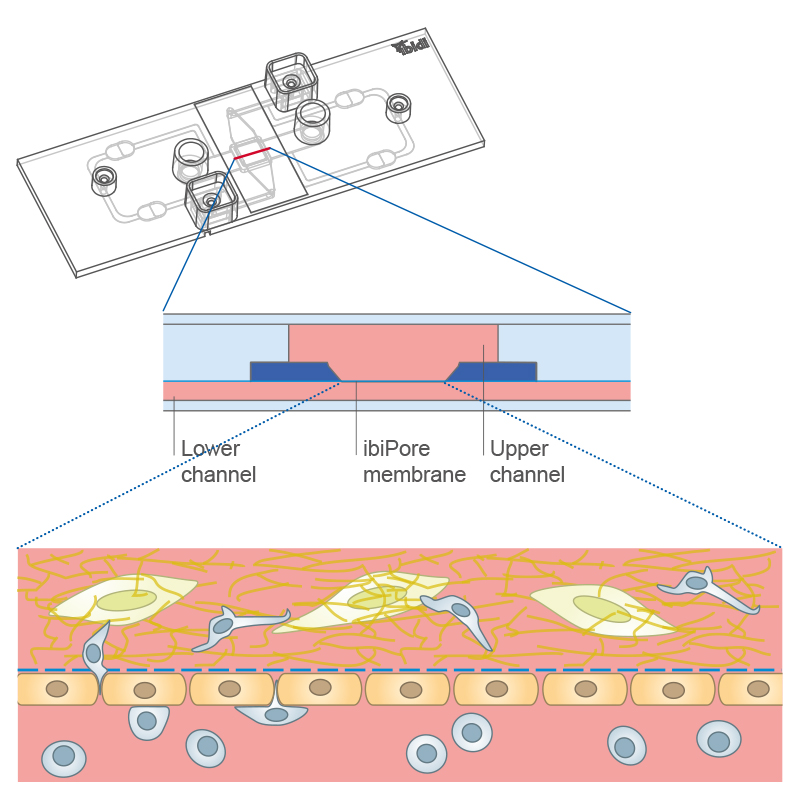
Transmigration of cells across a cell monolayer on the membrane into a 3D gel matrix with embedded cells using the µ-Slide ibiPore SiN.
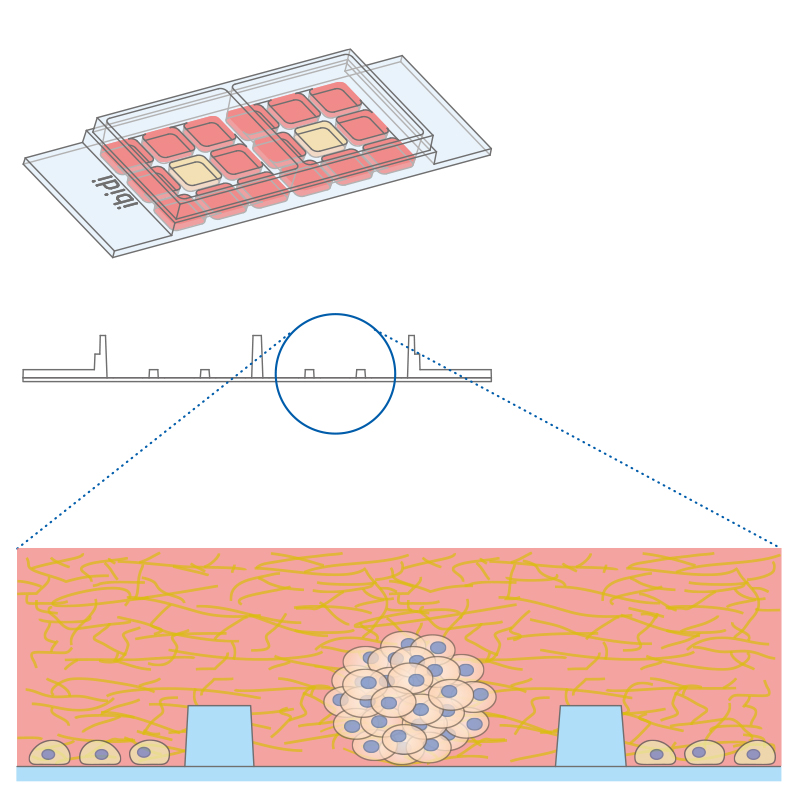
Co-cultivation of different cell types in a 3D matrix using the µ-Slide 2 Well Co-Culture.
ibidi Solutions for Co-Cultivation of Multiple 3D Cell Types
Selected Publications for Co-Cultivation of Multiple 3D Cell Types
The µ-Slide 2 Well Co-Culture and µ-Dish 35 mm, high were used to investigate the influence of Schwann cell-like spheroids on the formation of neurites by SH-SY5Y cells in co-culture assays.
Lin YJ, Lee YW, Chang CW, Huang CC. 3D Spheroids of Umbilical Cord Blood MSC-Derived Schwann Cells Promote Peripheral Nerve Regeneration. Front Cell Dev Biol. 2020;8:604946. doi:10.3389/fcell.2020.604946.
Read article
The µ-Plate 96 Well 3D was used for mono- and co-cultures of patient-derived pancreatic organoids with cancer-associated fibroblasts to analyze differential drug responses.
Schuth S, Le Blanc S, Krieger TG, Jabs J, Schenk M, Giese NA, Büchler MW, Eils R, Conrad C, Strobel O (2022) Patient-specific modeling of stroma-mediated chemoresistance of pancreatic cancer using a three-dimensional organoid-fibroblast co-culture system. J Exp Clin Cancer Res., 10.1186/s13046-022-02519-7.
Read article
Bioprinting of Complex 3D Tissue-Like Structures
Bioprinting is a highly automated and technology-intensive method that utilizes 3D printing to precisely position cells, biomaterials, and biochemicals to create tissue-like structures. This approach leverages the precision and automation of 3D printing systems to build complex, multi-layered tissue- and organ-like structures.
Bioprinting involves dispensing layers of bio-inks, which are composed of living cells and biocompatible materials, through a printing nozzle, guided by a rational design. These bio-inks can vary and include materials like hydrogels, which mimic the ECM. Post-printing, the printed structures undergo maturation to develop functional tissues. Bioprinting holds promise for various research areas, including tissue engineering, regenerative medicine, and basic research.

One of the main advantages of bioprinting is its precision and control, which is vital for creating complex tissue models. The process can be tailored for a wide variety of tissues and cell types, making it extremely versatile for various research applications. Furthermore, once a design is established, bioprinting allows for consistent and replicable creation of these complex structures, enhancing experimental reproducibility. On the downside, the technical demands and high costs of the equipment required make bioprinting available to only a few laboratories.
ibidi Solutions for Bioprinting of Complex 3D Tissues
Selected Publications for Bioprinting of Complex 3D Tissues
Bioprinted cultures were combind with dynamic flow culture, incorporating rationally designed perfusable vascular networks for functional maturation of 3D bioprinted liver constructs.
Tomov ML, Gil CJ, Cetnar A, et al. Engineering Functional Cardiac Tissues for Regenerative Medicine Applications. Curr Cardiol Rep. 2019;21(9):105. doi:10.1007/s11886-019-1178-9.
Read article
A pre-coated µ-Dish 35 mm, low Grid-500 was selected as a bioprinting support for a skin model used to study drug response.
Jeffries GDM, Xu S, Lobovkina T, Kirejev V, Tusseau F, Gyllensten C, Singh AK, Karila P, Moll L, Orwar O (2020) 3D micro-organisation printing of mammalian cells to generate biological tissues. Sci Rep., 10.1038/s41598-020-74191-w.
Read article
3D bioprinting properties of the Collagen Type I, Rat Tail hydrogel were systematically tested.
Stepanovska J, Supova M, Hanzalek K, Broz A, Matejka R. Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics. Biomedicines. 2021;9(9):1137. doi:10.3390/biomedicines9091137.
Read article
The µ-Dish 35 mm, low and the µ-Dish 35 mm, low Grid-500 were used for single-cell bioprinting.
Haylie R. Helms, Kody A. Oyama, Jason P. Ware, Stuart D. Ibsen, Luiz E. Bertassoni. Multiplex Single-Cell Bioprinting for Engineering of Heterogeneous Tissue Constructs with Subcellular Spatial Resolution, bioRxiv 2024.02.01.578499. 10.1101/2024.02.01.578499 (Preprint).
Read article
The µ-Dish 35 mm, low Grid-500 was used for bioprinting a cancer model that successfully recapitulates the intercellular communication via the assembly of functional tunneling nanotube (TNT)-like cell projections.
Herrada-Manchón H, Celada L, Rodríguez-González D, Alejandro Fernández M,Aguilar E, Chiara MD (2021) Three-dimensional bioprinted cancer models: Apowerful platform for investigating tunneling nanotube-like cell structures in complex microenvironments. Mater Sci Eng C Mater Biol Appl.,10.1016/j.msec.2021.112357.
Read article
Check out our Experimental Applications chapter for more examples!